
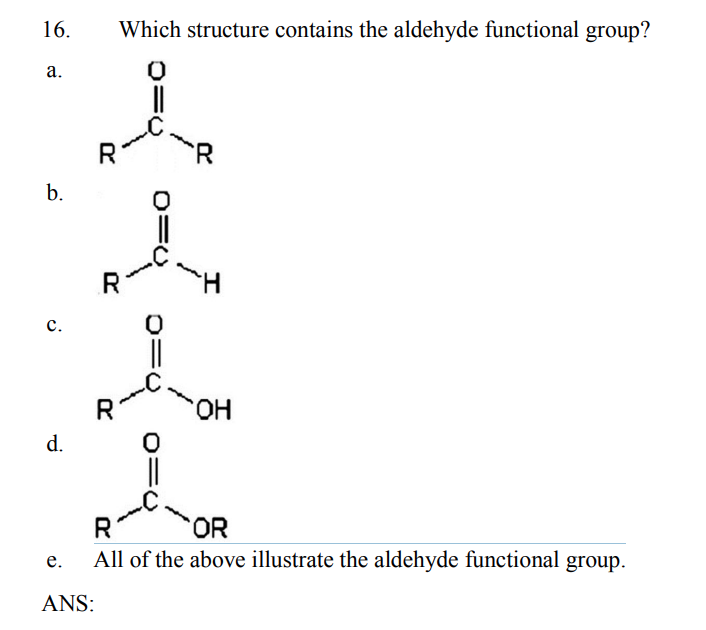
Substitution, followed by proton transfer, converts the leaving group into iodoform, which precipitates from the solution as a pale yellow solid. When a methyl ketone is mixed with iodine under aqueous alkaline conditions, iodine replaces each methyl hydrogen, making an excellent leaving group. We call this lack of reaction a negative result.Īnother useful reaction is used as the iodoform test for methyl ketones, which are ketones that have at least one methyl as a functional group. DNPH can also be used to distinguish alcohols and esters from aldehydes and ketones since DNPH does not react with alcohols or esters. However, aromatic ketones and aldehydes give a red-orange precipitate. When a non-aromatic ketone or aldehyde reacts with DNPH, the precipitate is yellow. This condensation reaction produces a hydrazone, which precipitates from the aqueous solution. In this reaction, 2,4-dinitrophenylhydrazine, or DNPH, attacks the carbonyl of an aldehyde or ketone in an aqueous acidic solution. One such reaction is the DNPH test, which is used to determine whether an aldehyde or ketone is aromatic. Certain reactions undergone by aldehydes and ketones can be used to distinguish them or identify their functional groups based on visible differences in the reaction's outcome. In contrast, a ketone has two carbon-based groups connected to the carbonyl carbon. The second group is either a hydrogen or a carbon-based group. An aldehyde has at least one hydrogen connected to the carbonyl carbon. Both possess a carbonyl group, which is a carbon double bonded to an oxygen. Aldehydes and ketones have a similar structure.


 0 kommentar(er)
0 kommentar(er)
